Ocean Acidification, A Global Threat To Marine Life
Introduction
ocean acidification : Ocean water becoming more acidic from greater concentration of hydrogen ions (H+).
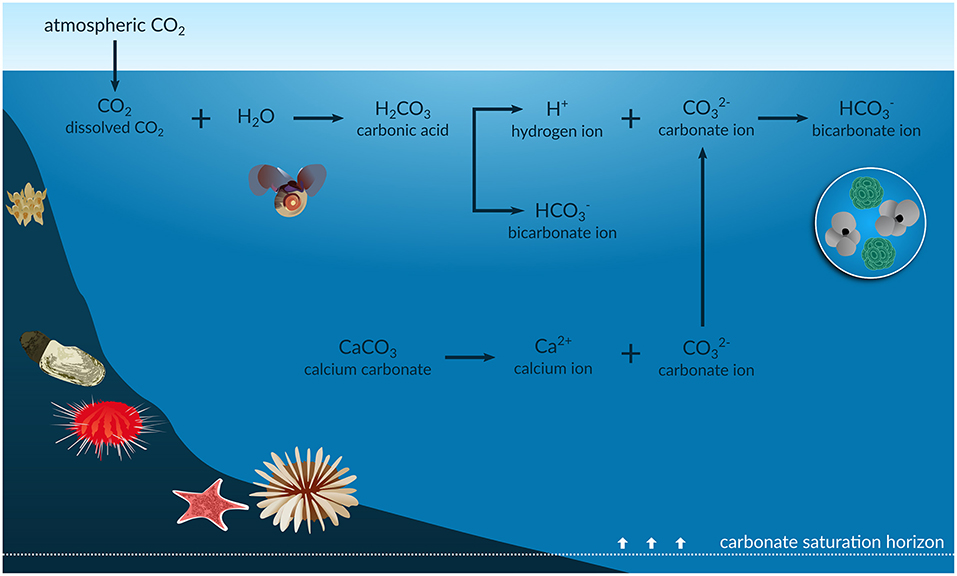
Carbon dioxide (CO2) combines with water (H2O) to create carbonic acid (H2CO3),
which then releases H+ ions to make the ocean more acidic.

Excess carbon dioxide : 30 billion tons per year, 84%, of CO2 are related to human activity. Natural processes remove some CO2; however if too much CO2 is released into the air, CO2 in our atmosphere increases.
CO2, carbon dioxide, dissolves into seawater : Carbon dioxide (CO2) mixes with water (H2O) to form carbonic acid. H2CO3, which is a weak acid.

industrial era : The transition to new manufacturing process that began between 1760 to 1840. The transition replaced hand or manual production to using machines, chemicals, steam, and coals.

CO2, carbon dioxide, dissolves into seawater : Carbon dioxide (CO2) mixes with water (H2O) to form carbonic acid. H2CO3, which is a weak acid.

ocean pH : Concentration of hydrogen ion (H+) in the ocean. It is a measure of how acidic or basic the ocean is. Lower pH is more acidic meaning greater concentration of positive H+ ions. Higher pH means the opposite, lower concentration H+ ions. Stable pH of the ocean is around 8.1.
dissolved chemicals from rocks : weathering and erosions dissolves rocks and minerals, which are then transported to river and eventually into ocean, impacting ocean chemistry
ocean pH : Concentration of hydrogen ion (H+) in the ocean. It is a measure of how acidic or basic the ocean is. Lower pH is more acidic meaning greater concentration of positive H+ ions. Higher pH means the opposite, lower concentration H+ ions. Stable pH of the ocean is around 8.1.
ocean pH : Concentration of hydrogen ion (H+) in the ocean. It is a measure of how acidic or basic the ocean is. Lower pH is more acidic meaning greater concentration of positive H+ ions. Higher pH means the opposite, lower concentration H+ ions. Stable pH of the ocean is around 8.1.
ocean pH : Concentration of hydrogen ion (H+) in the ocean. It is a measure of how acidic or basic the ocean is. Lower pH is more acidic meaning greater concentration of positive H+ ions. Higher pH means the opposite, lower concentration H+ ions. Stable pH of the ocean is around 8.1.
ocean acidification : Ocean water becoming more acidic from greater concentration of hydrogen ions (H+).
Carbon dioxide (CO2) combines with water (H2O) to create carbonic acid (H2CO3),
which then releases H+ ions to make the ocean more acidic.

shells already dissolving in the more acidic seawater : In a lab expirement, a sea butterfly shell placed in seawater with increased acidity was found to slowly dissolve over a 45 day period.
estuaries : estuaries and their surrounding wetlands are bodies of water usually found where rivers meet the sea.

ocean acidification : Ocean water becoming more acidic from greater concentration of hydrogen ions (H+).
Carbon dioxide (CO2) combines with water (H2O) to create carbonic acid (H2CO3),
which then releases H+ ions to make the ocean more acidic.

the term "ocean acidification" was first coined : The 2003 article published in Nature finds that CO2 from fossil fuels may result in larger pH changes than any geological record of the past 300 million years.
ocean pH : Concentration of hydrogen ion (H+) in the ocean. It is a measure of how acidic or basic the ocean is. Lower pH is more acidic meaning greater concentration of positive H+ ions. Higher pH means the opposite, lower concentration H+ ions. Stable pH of the ocean is around 8.1.
Acidification Chemistry
ocean acidification : Ocean water becoming more acidic from greater concentration of hydrogen ions (H+).
Carbon dioxide (CO2) combines with water (H2O) to create carbonic acid (H2CO3),
which then releases H+ ions to make the ocean more acidic.

CO2, carbon dioxide, dissolves into seawater : Carbon dioxide (CO2) mixes with water (H2O) to form carbonic acid. H2CO3, which is a weak acid.

lower pH : The uptake of CO2 in the ocean decreases pH levels, making the ocean more acidic and effecting marine life.
A More Acidic Ocean

ocean pH : Concentration of hydrogen ion (H+) in the ocean. It is a measure of how acidic or basic the ocean is. Lower pH is more acidic meaning greater concentration of positive H+ ions. Higher pH means the opposite, lower concentration H+ ions. Stable pH of the ocean is around 8.1.
NOAA PMEL Carbon Program : Government researchers whose mission is to understand the changing chemistry of the oceans and impact on marine ecosystems.
CO2, carbon dioxide, dissolves into seawater : Carbon dioxide (CO2) mixes with water (H2O) to form carbonic acid. H2CO3, which is a weak acid.

When water (H 2 O) and CO 2 mix, they combine to form carbonic acid (H 2 CO 3 ). Carbonic acid is weak compared to some of the well-known acids that break down solids, such as hydrochloric acid (the main ingredient in gastric acid, which digests food in your stomach) and sulfuric acid (the main ingredient in car batteries, which can burn your skin with just a drop). The weaker carbonic acid may not act as quickly, but it works the same way as all acids: it releases hydrogen ions (H + ), which bond with other molecules in the area.
lower pH : The uptake of CO2 in the ocean decreases pH levels, making the ocean more acidic and effecting marine life.
lower pH : The uptake of CO2 in the ocean decreases pH levels, making the ocean more acidic and effecting marine life.
ocean pH : Concentration of hydrogen ion (H+) in the ocean. It is a measure of how acidic or basic the ocean is. Lower pH is more acidic meaning greater concentration of positive H+ ions. Higher pH means the opposite, lower concentration H+ ions. Stable pH of the ocean is around 8.1.
ocean pH : Concentration of hydrogen ion (H+) in the ocean. It is a measure of how acidic or basic the ocean is. Lower pH is more acidic meaning greater concentration of positive H+ ions. Higher pH means the opposite, lower concentration H+ ions. Stable pH of the ocean is around 8.1.
logarithm : logarithm counts the number of occurrences of the same factor in repeated multiplication; e.g. since 1000 = 10 × 10 × 10 = 10^3, the "logarithm base 10" of 1000 is 3, or log10 (1000) = 3. Similarly, log10 (10,000) = 4. The difference between 3 and 4 is a factor of 10 times the amount.
CO2, carbon dioxide, dissolves into seawater : Carbon dioxide (CO2) mixes with water (H2O) to form carbonic acid. H2CO3, which is a weak acid.

ocean pH : Concentration of hydrogen ion (H+) in the ocean. It is a measure of how acidic or basic the ocean is. Lower pH is more acidic meaning greater concentration of positive H+ ions. Higher pH means the opposite, lower concentration H+ ions. Stable pH of the ocean is around 8.1.
Why Acidity Matters

Many chemical reactions, including those that are essential for life, are sensitive to small changes in pH. In humans, for example, normal blood pH ranges between 7.35 and 7.45. A drop in blood pH of 0.2-0.3 can cause seizures, comas, and even death. Similarly, a small change in the pH of seawater can have harmful effects on marine life, impacting chemical communication, reproduction, and growth.
hydrogen ions bond with carbonate : hydrogen ions steal carbonate (CO3--) to form bircarbonate (HCO3-). That leaves fewer carbonate (CO3--) to react with Calcium to create shells (calcium carbonate, CaCO3) for reefs.
calcium carbonate : A thin layer of crystals that forms the basis of coral's skeleton.
calcium carbonate : A thin layer of crystals that forms the basis of coral's skeleton.
CO2, carbon dioxide, dissolves into seawater : Carbon dioxide (CO2) mixes with water (H2O) to form carbonic acid. H2CO3, which is a weak acid.

calcium carbonate : A thin layer of crystals that forms the basis of coral's skeleton.
hydrogen ions bond with carbonate : hydrogen ions steal carbonate (CO3--) to form bircarbonate (HCO3-). That leaves fewer carbonate (CO3--) to react with Calcium to create shells (calcium carbonate, CaCO3) for reefs.

bicarbonate : Anion with formula HCO3-. Bicarbonates are less stable than carbonate ion (CO3--). Ocean acidifcation's H+ ions steals carbonate ion (CO3--) to create bicarbonate(HCO3-). Carbonate ions ((CO3--) are needed by corals to form their skeleton. The key difference between carbonate and bicarbonate is that the carbonate ion has -2 electrical charge whereas, the bicarbonate has -1 electrical charge. Few corals can build skeleton using bicarbonate instead of carbonate ion; however, vast majority of corals use carbonate ions, not bicarbonate.

bicarbonate : Anion with formula HCO3-. Bicarbonates are less stable than carbonate ion (CO3--). Ocean acidifcation's H+ ions steals carbonate ion (CO3--) to create bicarbonate(HCO3-). Carbonate ions ((CO3--) are needed by corals to form their skeleton. The key difference between carbonate and bicarbonate is that the carbonate ion has -2 electrical charge whereas, the bicarbonate has -1 electrical charge. Few corals can build skeleton using bicarbonate instead of carbonate ion; however, vast majority of corals use carbonate ions, not bicarbonate.

calcium carbonate : A thin layer of crystals that forms the basis of coral's skeleton.
hydrogen ions bond with carbonate : hydrogen ions steal carbonate (CO3--) to form bircarbonate (HCO3-). That leaves fewer carbonate (CO3--) to react with Calcium to create shells (calcium carbonate, CaCO3) for reefs.

CO2, carbon dioxide, dissolves into seawater : Carbon dioxide (CO2) mixes with water (H2O) to form carbonic acid. H2CO3, which is a weak acid.

Impacts on Ocean Life
ocean pH : Concentration of hydrogen ion (H+) in the ocean. It is a measure of how acidic or basic the ocean is. Lower pH is more acidic meaning greater concentration of positive H+ ions. Higher pH means the opposite, lower concentration H+ ions. Stable pH of the ocean is around 8.1.
Coral Reefs

model for a more acidic future ocean : Normanby Island in Papua New Guinea shows that under acidic condition massive brownish boulder corals colonize the sea floor instead of the usually beautiful, lively, colorful and complex corals.

calcium carbonate : A thin layer of crystals that forms the basis of coral's skeleton.
forming complex reefs : Coral reefs are the most diverse of all marine ecosystems. They teem with life, with perhaps 25% of all ocean species depending on reefs for food and shelter. They are often called the rainforests of the sea.

limit coral growth : Lower ocean pH (increased H+ ions) decrease coral calcification rates significantly. The acidification lowers the concentration of carbonate ions in seawater, making it more difficult for corals to build their calcium carbonate skeletons.
animals that drill into : Ocean acidification provides advantages to ocean sponges that breaks down coral reefs. The acidification and increased temperature help sponges degrade coral skeleton faster.

coral larvae development : “The coral larvae normally have this amazing ability to settle on one particular type of rock-like seaweed called Titanoderma. This stony seaweed is a safe haven for young corals...yet, as levels of ocean acidification increased, the coral larvae avoided this seaweed and started to settle absolutely anywhere.” says research scientist Christopher Doropoulos
plankton : The word “plankton” comes from the Greek for “drifter” or “wanderer.” An organism is considered plankton if it is carried by tides and currents, and cannot swim well enough to move against these forces. Plankton are usually microscopic, often less than one inch in length, but they also include larger species like some crustaceans and jellyfish.

larvae in acidic water had more trouble finding a good place to settle : Increasingly acidic conditions in the ocean appear to have a dramatic effect on the ability of baby corals to sense their surroundings.

bicarbonate : Anion with formula HCO3-. Bicarbonates are less stable than carbonate ion (CO3--). Ocean acidifcation's H+ ions steals carbonate ion (CO3--) to create bicarbonate(HCO3-). Carbonate ions ((CO3--) are needed by corals to form their skeleton. The key difference between carbonate and bicarbonate is that the carbonate ion has -2 electrical charge whereas, the bicarbonate has -1 electrical charge. Few corals can build skeleton using bicarbonate instead of carbonate ion; however, vast majority of corals use carbonate ions, not bicarbonate.

return to normal skeleton-building activities : Some corals can survive acidic condition and resume growth when normal conditions are reintroduced
On reefs in Papua New Guinea : Normanby Island in Papua New Guinea shows that under acidic condition massive brownish boulder corals colonize the sea floor Instead of the usually beautiful, lively, colorful and complex corals.

coral bleaching : Tiny plant-like organisms called zooxanthellae live in the tissues of some corals. These microscopic algae capture sunlight and convert it into energy, just like plants, to provide essential nutrients to the corals. But under high temperature conditions, they will die or leave their host—a process known as bleaching.

Looking to the Future
ocean acidification : Ocean water becoming more acidic from greater concentration of hydrogen ions (H+).
Carbon dioxide (CO2) combines with water (H2O) to create carbonic acid (H2CO3),
which then releases H+ ions to make the ocean more acidic.

CO2, carbon dioxide, dissolves into seawater : Carbon dioxide (CO2) mixes with water (H2O) to form carbonic acid. H2CO3, which is a weak acid.